Consider the graph between compressibility factor Z and pressure P
4.6 (281) In stock

Z1 means force of attraction dominating ie a is considerable b can be negligible at low temperature and low pressure Lower is the value of Z easier is the process of liquification
The compressibility factor is actually a factor that corrects the actual value of the gas versus the ideal gas. Let us learn and understand this concept.
Watch this video to understand the behaviour of real gases with the help of the compressibility factor. This is an important topic for JEE main.
What is the compressibility factor, and how does it vary with an increase in temperature and pressure? Watch this video to get the answer. This is an importa

thermodynamics - Variation of compressiblity factor with temperature - Chemistry Stack Exchange

Consider the graph between compressibility factor Z and pressure P

Praveen-Fl (22-23) MCT - 1, PDF, Acceleration
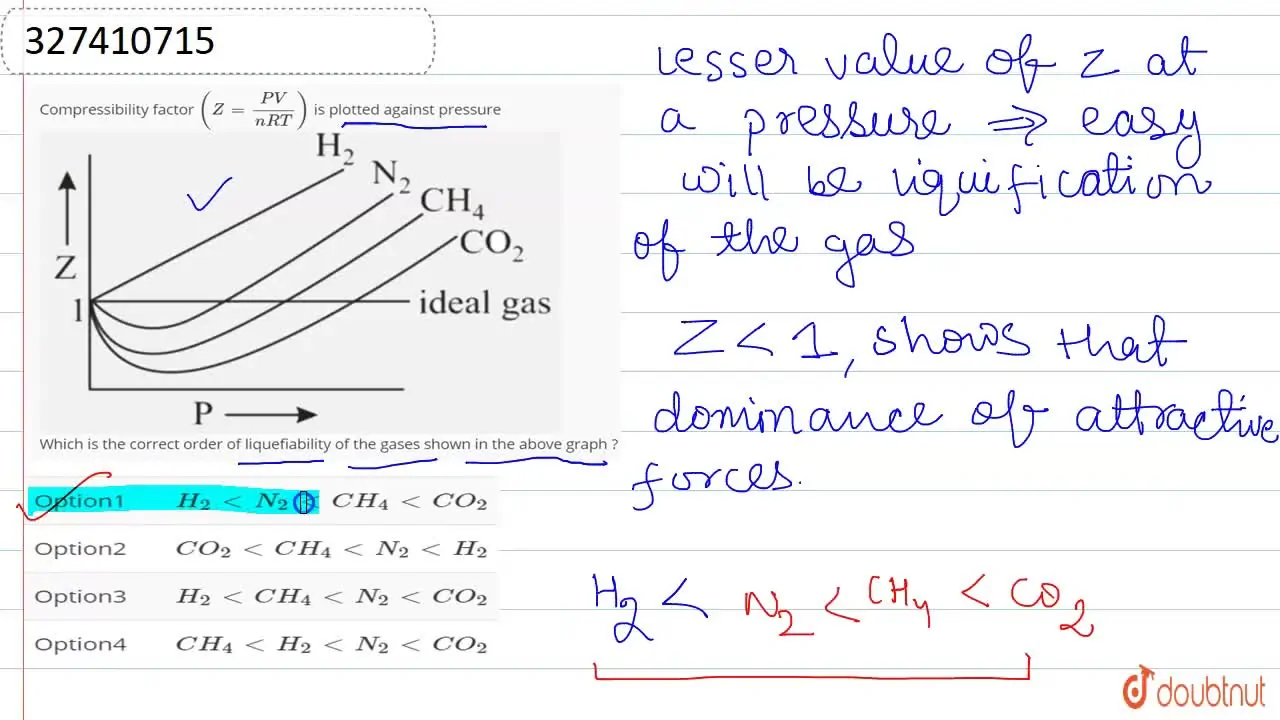
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure
The effect of Pressure on Temperature-Compressibility Factor diagram

PDF) ACT- All Goa Chemistry Quiz - Std.XI - December 2017actgoa.weebly.com/uploads/3/7/2/3/37238293/act_xi_20171 ACT- All Goa Chemistry Quiz - Std.XI - December – 2017 Date: 18/12/17
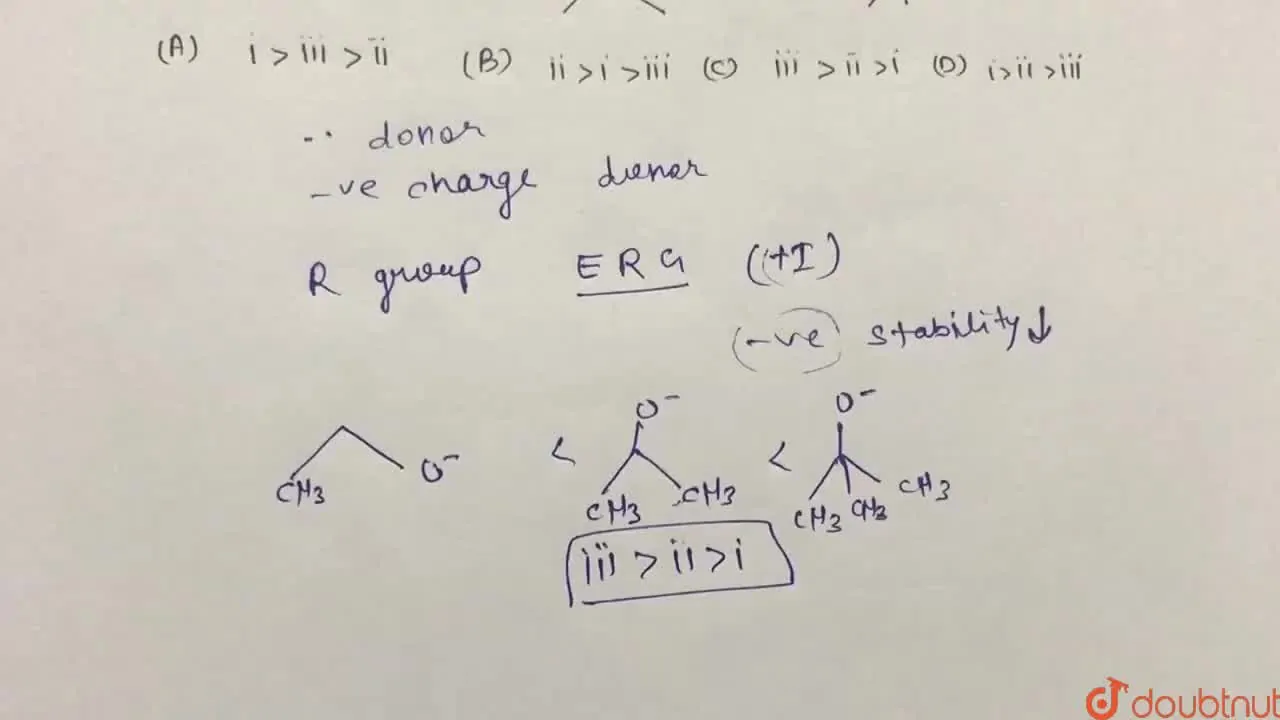
Determine the order of basic stregth of the given molecules
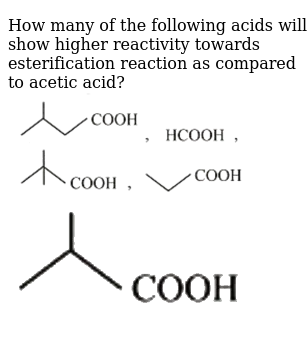
How many of the following acids will show higher reactivity towards es
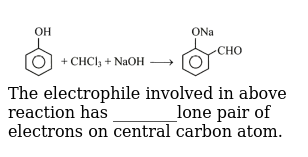
The electrophile involved in above reaction has lone pair of electrons

Compressibility factor of benzene vapor along its saturation curve. P i

Praveen-Fl (22-23) MCT - 1, PDF, Acceleration

PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H

Telugu] The variation of compressibility factor (Z) with pressure (p

Energies, Free Full-Text

Consider the graph between compressibility factor Z and pressure P
Compressibility factor (Z) for a van der Waals real gas at
Math cad compressibility factor, z, of real gas using the redlich
Real gas z-Factor chart [2] Download Scientific Diagram
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure
 Is Shein's $50 Million Fund To Tackle Clothing Waste A Good Thing, Or Just Greenwashing?
Is Shein's $50 Million Fund To Tackle Clothing Waste A Good Thing, Or Just Greenwashing? Core Exercises to Reduce Your Abdominal Separation - Perfect Pelvic Floor
Core Exercises to Reduce Your Abdominal Separation - Perfect Pelvic Floor- Girls 4-12 Jumping Beans® Leggings
 STRETCH IS COMFORT Women's Cotton Strapless Long Tube Top, Made in The USA
STRETCH IS COMFORT Women's Cotton Strapless Long Tube Top, Made in The USA Black Elsie Top High-Construction Bikini Bralette – Sunsets Inc.
Black Elsie Top High-Construction Bikini Bralette – Sunsets Inc. Equipo Fashion for Men
Equipo Fashion for Men
