gas laws - How to find the temperature relationship between the
5 (111) In stock

The following graph denotes the variation of the compressibility factor (Z) with pressure at different temperatures for a real gas. Simply each of the curves represents an isotherm. Now, suppose w

Solved The ideal gas law describes the relationship among

Ideal–Universal Gas Law
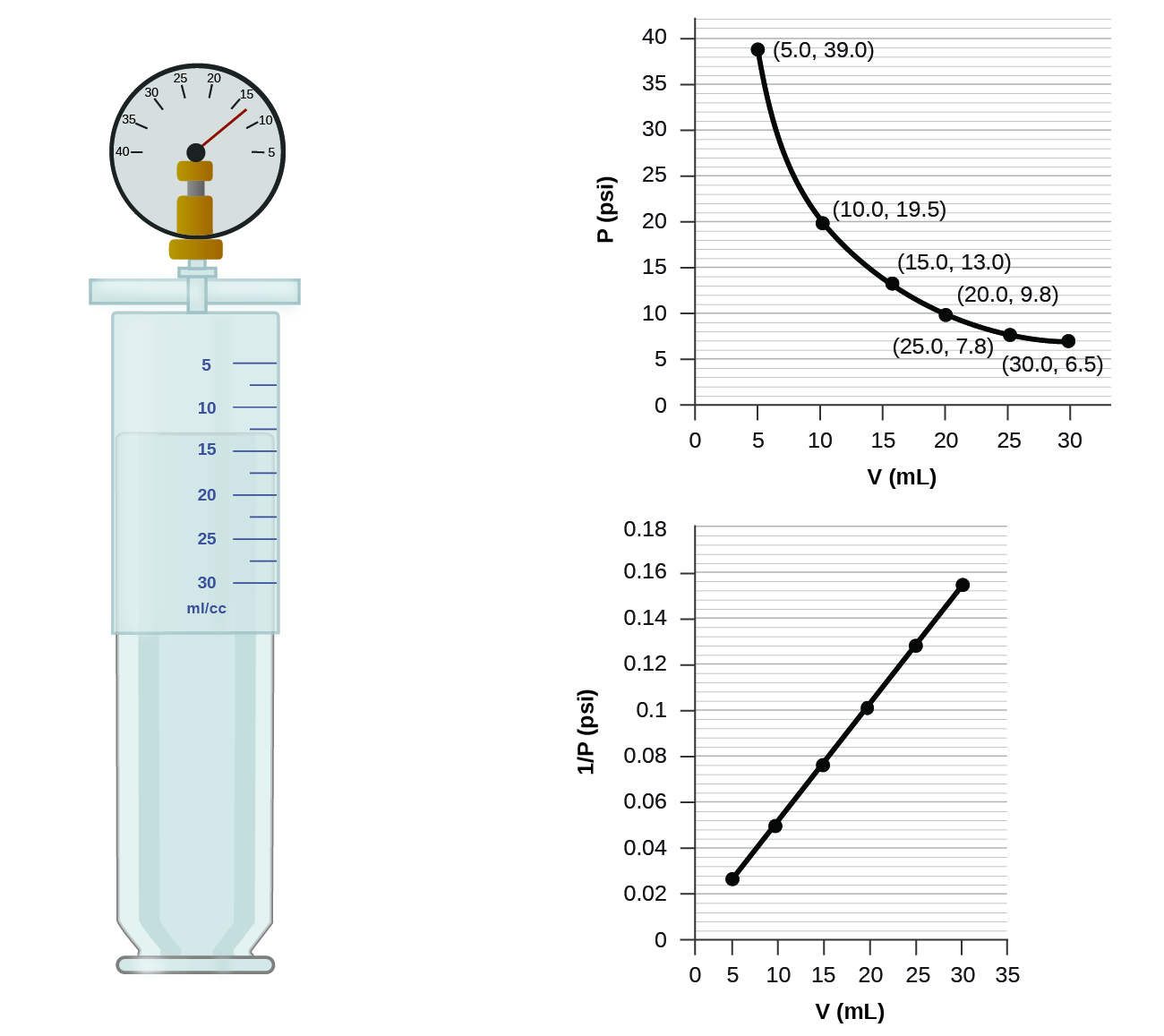
8.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law – Chemistry

12.2 First law of Thermodynamics: Thermal Energy and Work
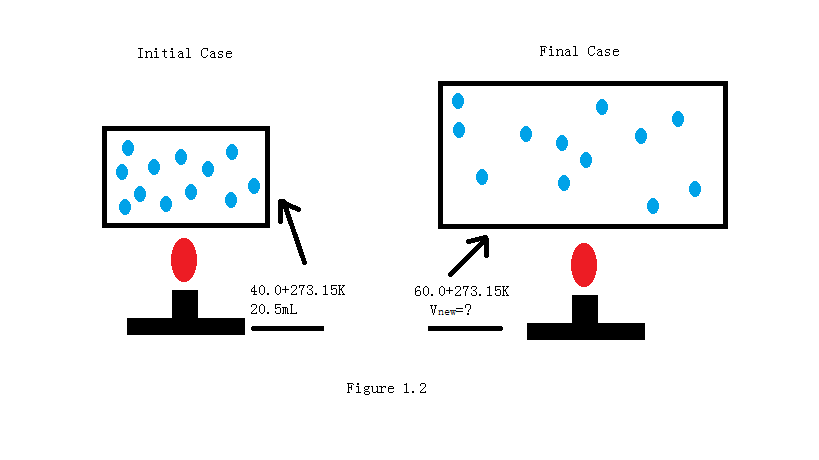
Gas Laws - Overview - Chemistry LibreTexts
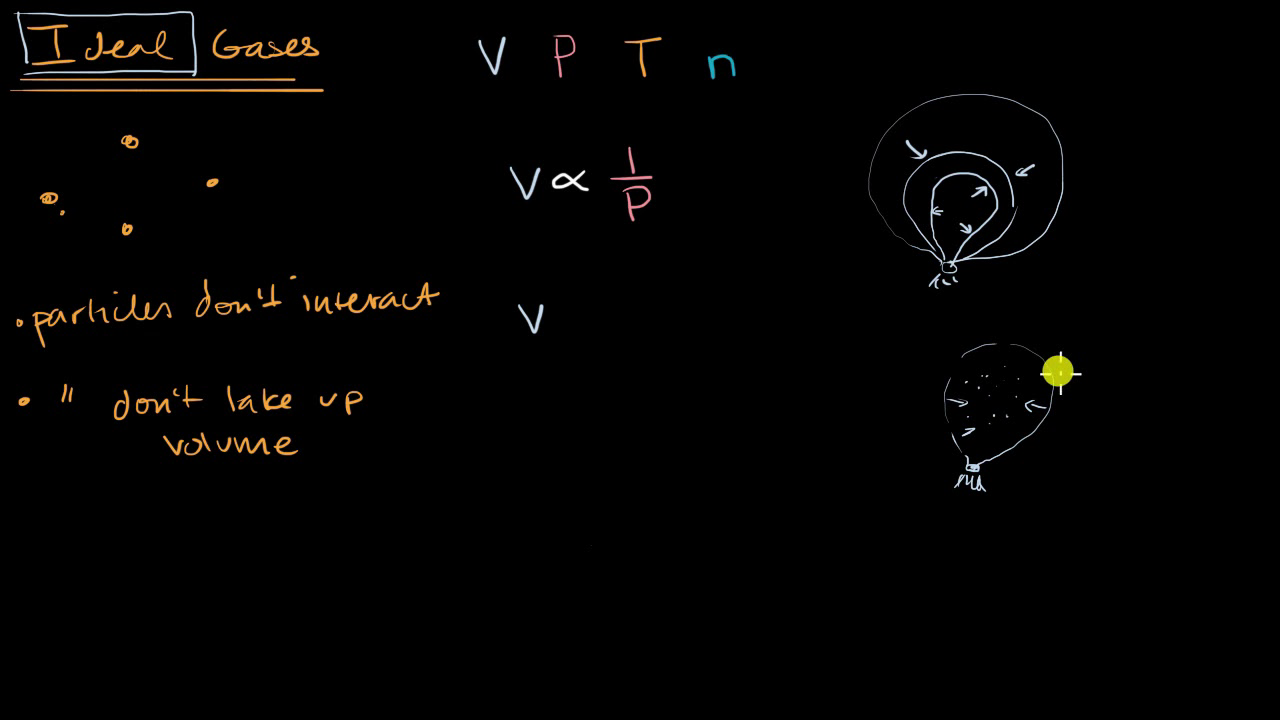
The ideal gas law (PV = nRT) (video)

Question Video: Finding the Initial Temperature of a Gas Using Charles' Law
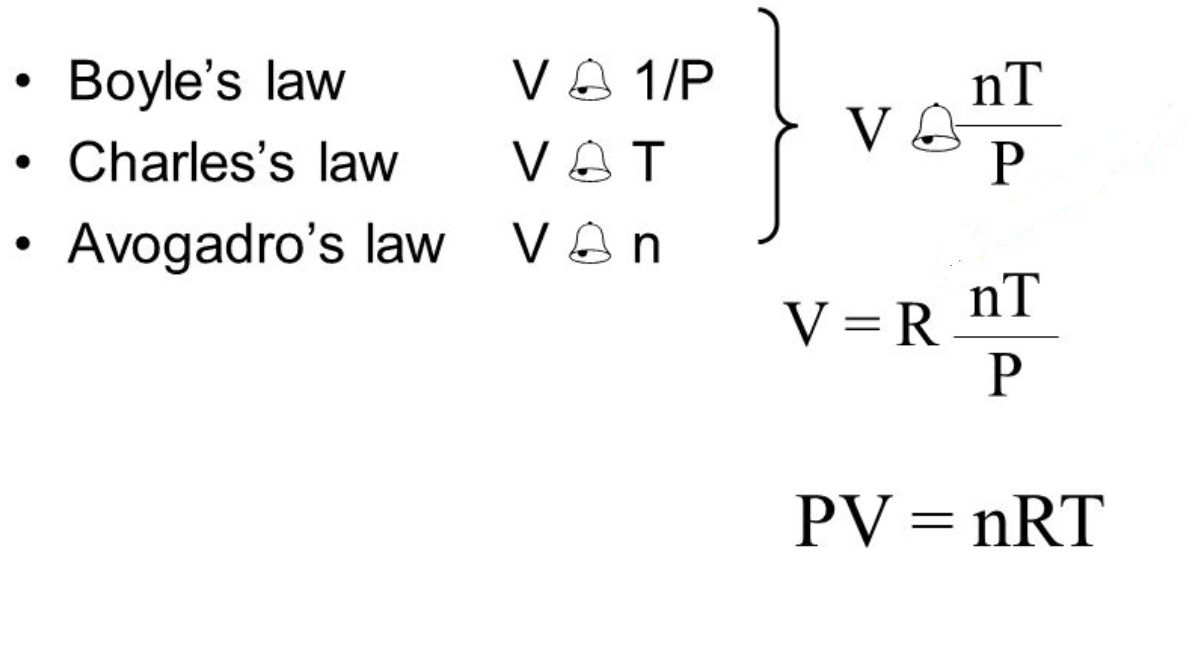
Gas Laws

Question Video: Determining the Relationship between Temperature and Number of Moles of an Ideal Gas
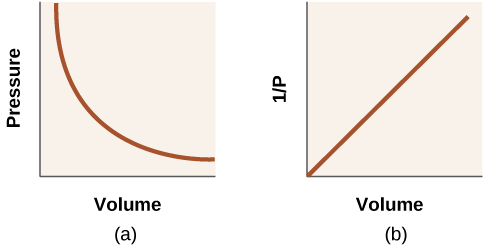
Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law – Chemistry
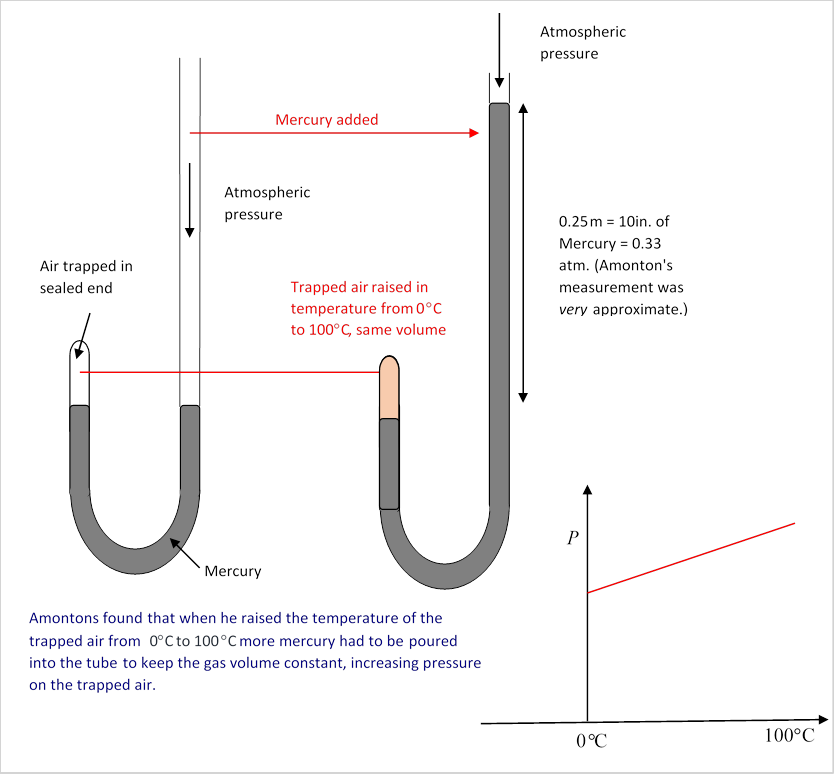
Gas Law and Avogadro

PV=nRT - Use the Ideal Gas Law Ideal gas law, Gas constant, Chemistry 101

Combined Gas Law - Definition, Formula, Examples
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
New explicit correlation for the compressibility factor of natural
Real Gases vs Ideal Gases & the Compressibility Factor
The role of the compressibility factor Z in describing the volumetric behavior of gases
 Vince Camuto Full-figure T-shirt Bra
Vince Camuto Full-figure T-shirt Bra Beginner Machine Embroidery Project #2- Free standing lace
Beginner Machine Embroidery Project #2- Free standing lace DAILY GRIND LEGGINGS
DAILY GRIND LEGGINGS Fabletics Pastel PowerHold Define High Waisted Crop Leggings Size L
Fabletics Pastel PowerHold Define High Waisted Crop Leggings Size L Order Form Template, Order Form Template Editable, Sign up Form, Order Form Template for Crafters, Booking Form, Order Form Printable C01
Order Form Template, Order Form Template Editable, Sign up Form, Order Form Template for Crafters, Booking Form, Order Form Printable C01 Pink Caviar Pride Pool Party Los Angeles 2024
Pink Caviar Pride Pool Party Los Angeles 2024