What is the value of compressibility factor in terms of vander
4.6 (684) In stock
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons-an t at different conditions of pressure and volume-Why is Z-1 for H2 and He gas
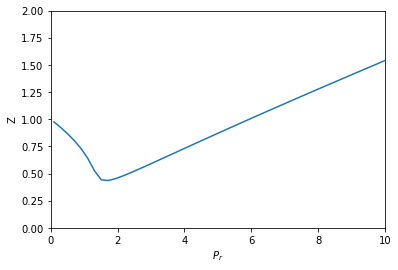
Compressibility factor variation from the van der Waals equation by three different approaches

If Z is a compressibility factor, van der Waals equation at low pressure ..

Torateal gas, the compressibility factor Z has different whues
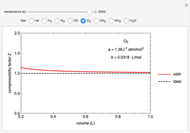
Compressibility Factors for van der Waals Gases - Wolfram Demonstrations Project

If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as

Van der Waals Equation, Definition & Examples - Lesson
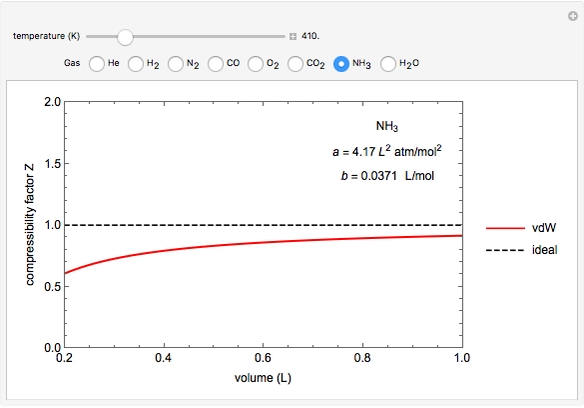
Compressibility Factors for van der Waals Gases - Wolfram Demonstrations Project

plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

Gas compressibility factor Z: Ideal gas vs Real gas

Gas Compressibility Factor and Control Valve Sizing

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

SOLVED: (a) State the van der Waals gas equation, defining all its terms and their units [3 MARKS] (b) Derive an expression for the excluded volume of a gas (per mole of

b 26. The compressibility factor 1 mole of a van der Waal's gas Boyle temperature is 1+ VIV-yo) Find the value of x + y. tronarding the van property?
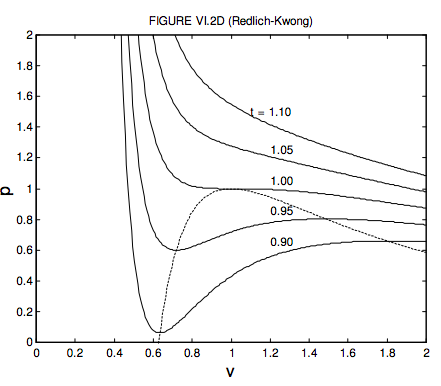
6.3: Van der Waals and Other Gases - Physics LibreTexts
Compressibility Factor Z for sub-critical pressures for Lee
In the following compressibility factor Z vs pressure graph at 300
Compressibility factor Z for sub-critical pressures in a 'one-cell
 Aston Martin raises £200m to fund new DBX crossover
Aston Martin raises £200m to fund new DBX crossover 2PCS Aquatic Dumbbells Water Weights,Water Dumbell Pool Resistance Water Fitness Equipment Foam Dumbbell,Aqua Fitness Swimming Barbells,Water Aerobics
2PCS Aquatic Dumbbells Water Weights,Water Dumbell Pool Resistance Water Fitness Equipment Foam Dumbbell,Aqua Fitness Swimming Barbells,Water Aerobics White - button down oxford shirt – GROUNDERBKK
White - button down oxford shirt – GROUNDERBKK 4mm x 40mm Stainless Steel D Ring
4mm x 40mm Stainless Steel D Ring Avidlove Sexy Lingerie Set for Women Lace Bra and India
Avidlove Sexy Lingerie Set for Women Lace Bra and India Hesxuno Sexy Lingerie Set Ladies Lingerie Set Sexy Lace Sling Bra and Panties Summer Thin Lingerie Set
Hesxuno Sexy Lingerie Set Ladies Lingerie Set Sexy Lace Sling Bra and Panties Summer Thin Lingerie Set