If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as
4.9 (575) In stock


Class Xi States of Matter, PDF, Gases
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

Jee main-2014-solution-code-h-english

If Z is compressibility factor, vander Waals equation low pressure can be written as
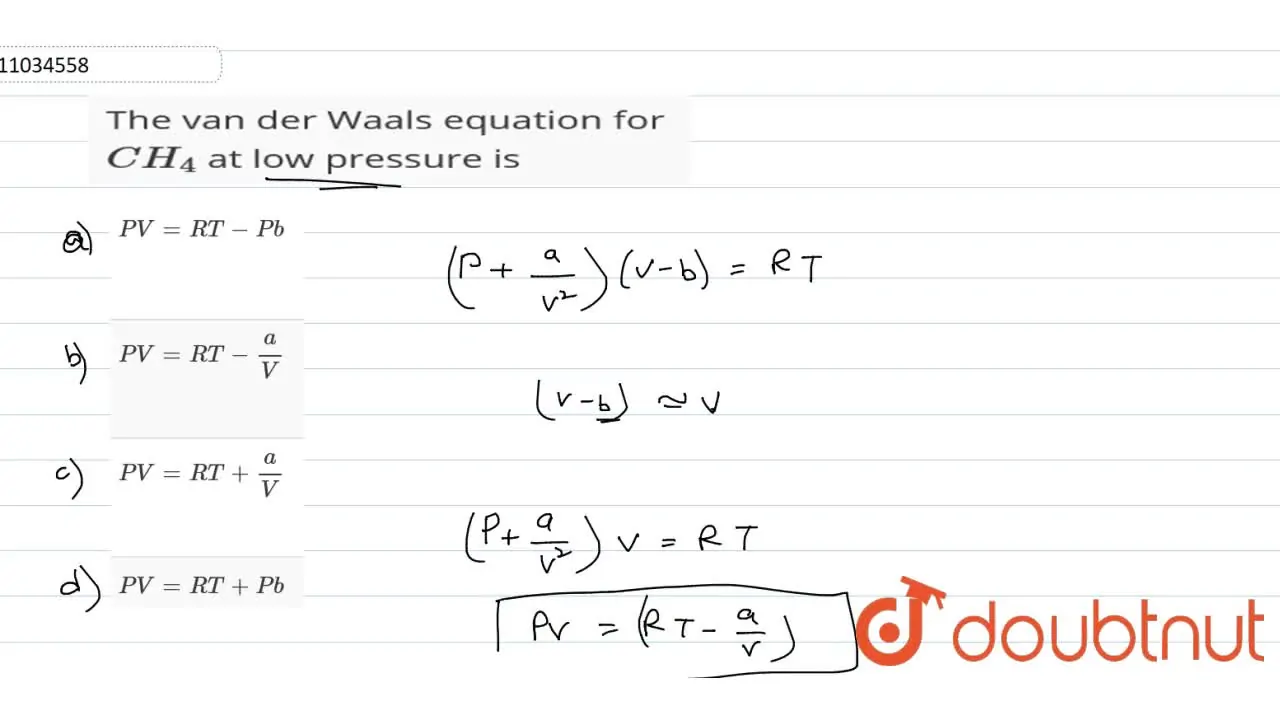
The van der Waals equation for CH(4) at low pressure is

Given Vapour pressure of H 2 O at 300 K is 3170 Pa R 8314 JK 1 mol 1 2010 A 127

Chemistry Edge - Target NEET/JEE 2021

SOLVED: The fugacity of a van der Waals gas can be determined using the expression for the compressibility factor Z. The expression for fugacity of a van der Waals gas is given

At low pressures, the van der Waals equation is written as [P+(a)/(V^(

If Z is a compressibility factor, vander Waals equation low pressure can be written as [JEEN (0)2=1 Rang (1) Z= 1 + RT Pb (2) Z 2)2=1= = 1 - 2= (3) Z = 1 - 42=1 (4)Z = 1 + VRT
At high temperature and low pressure van der Waals equation can be expressed as?

Jee main-2014-solution-code-h-english

Gaseous State Questions for JEE exam - Free Online All questions of Gaseous State - Chapter-wise Questions of JEE

Which of these are correct? A) Z, compressibility factor, low pressure can be written as z = B) Z, low pressure can be written as z = 1 + P C) Z
Compressibility Factor of Carbon Dioxide - Maple Application Center
Class Notes on Compressibility of a Real Gas, CH 417, Study notes Physical Chemistry
 TKD-127, Copyright © 2012 Alexandre Calixto, TKD Brasil
TKD-127, Copyright © 2012 Alexandre Calixto, TKD Brasil- Playtex Intimates on Instagram: “Love this bra, have been
 FSLE Tapered High Waisted Capris For Women Spring Next Petite
FSLE Tapered High Waisted Capris For Women Spring Next Petite Buy Brida Barsa - Cotton Full Coverage Minimiser Bra - Non Padded, Non Wired Online In India At Discounted Prices
Buy Brida Barsa - Cotton Full Coverage Minimiser Bra - Non Padded, Non Wired Online In India At Discounted Prices Miracle-Gro Moisture Control Potting Mix
Miracle-Gro Moisture Control Potting Mix Pink Wrap V Neck Floral Maxi Dress – Emmeline's Fashion
Pink Wrap V Neck Floral Maxi Dress – Emmeline's Fashion
